Novavax Says Its COVID-19 Vaccine Prevents Moderate to Severe Infections
The biotech firm says its vaccine also works against variants. Wayne State University was one of the testing sites.
A fourth COVID-19 vaccine could be on its way soon.
Novavax, a biotech firm in Delaware, says its vaccine is highly effective at preventing moderate to severe infections caused by the SARS-CoV-2 virus and some of its variants. The company is expected to seek emergency authorization to begin distributing the vaccine in the United States.
“Go ahead and get vaccinated with what’s available.” –Dr. Elizabeth Secord, principal investigator for Wayne State University’s participation in the Novavax COVID-19 vaccine trial
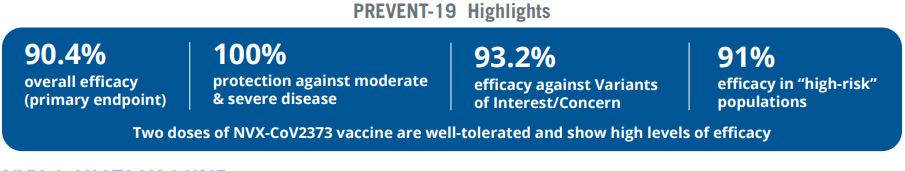
Novavax published data of almost 30,000 trial subjects in the U.S. and Canada. Two-thirds got the vaccine, while the rest were given placebos. The study found that the vaccine had an overall efficacy rate of 90% and was 100% effective against moderate or severe disease in people who got the vaccine.
Wayne State University was one of 113 Novavax COVID-19 vaccine test sites in the U.S. Dr. Elizabeth Secord is the principal investigator at the WSU site. She says 158 people participated in the Detroit trial.

“We had some drop out who decided they had to get other vaccines,” she says. “So at last count, we had about 135 who have gone through the whole vaccine process and are continuing in this study.”
Secord says unlike the Pfizer and Moderna COVID-19 vaccines, the Novavax vaccine does not use messenger RNA. It also does not have to be stored at subzero temperatures.
“It is a two-dose vaccine, and can be kept in a refrigerator,” she says.
While only mild side effects were reported in the Novavax trial, Secord says there’s no guarantee something more serious won’t turn up later.
“With the other vaccines in the initial trials, there weren’t serious side effects, but when they were rolled out to hundreds of thousands of people, there were some more serious side effects,” she says.
Related: WDET: Wayne State Seeks Volunteers For COVID-19 Vaccine Trial
NPR: The Results Are In For Novavax’s Vaccine And They Are Impressive
NPR: How Novavax Compares To Other COVID-19 Vaccines
Side effects such as blood clots have been reported in vaccinated people, but are extremely rare. Health experts say the benefits of being vaccinated far outweigh the risks. Secord agrees and encourages people to get vaccinated as soon as possible.
“I don’t think people should wait for a particular vaccine,” she says. “Go ahead and get vaccinated with what’s available.”
Listen: How the Novavax vaccine is different than Pfizer and Moderna’s vaccines.
Trusted, accurate, up-to-date
WDET is here to keep you informed on essential information, news and resources related to COVID-19.
This is a stressful, insecure time for many. So it’s more important than ever for you, our listeners and readers, who are able to donate to keep supporting WDET’s mission. Please make a gift today.
